Young Yeap Blogs

YOUNG EAP BLOG – COVID-19 SERIES (#8) | THE COVID-19 RESPONSE IN AUSTRIA
COVID-19 SERIES (#8) The COVID-19 response in Austria. On 25th of February 2020, Austria confirmed the first two cases of COrona VIrus Disease 2019 (COVID-19), a couple from Lombardy, Italy. (1) In the following days more patients were tested positive…

YOUNG EAP BLOG – COVID-19 SERIES (#9) | SARS-COV-2 IN GERMANY
COVID-19 SERIES (#9) SARS-CoV-2 in Germany. The first case of COVID-19 in Germany was reported on 28th of January 2020. A man from Bavaria (Starnberg) had work-related contact with a Chinese colleague who is assumed to be the source of his infection….

RCPCH BLOG – COVID-19 SERIES (#6) | MESSAGE FROM ROYAL COLLEGE OF PAEDIATRICS AND CHILD HEALTH PRESIDENT
COVID-19 SERIES (#6) Message from the Royal College of Paediatrics and Child Health President. MESSAGE FROM RCPCH PRESIDENT, 24 APRIL Professor Russell Viner’s weekly blog to members looks at how we protect and plan delivery of crucial paediatric…
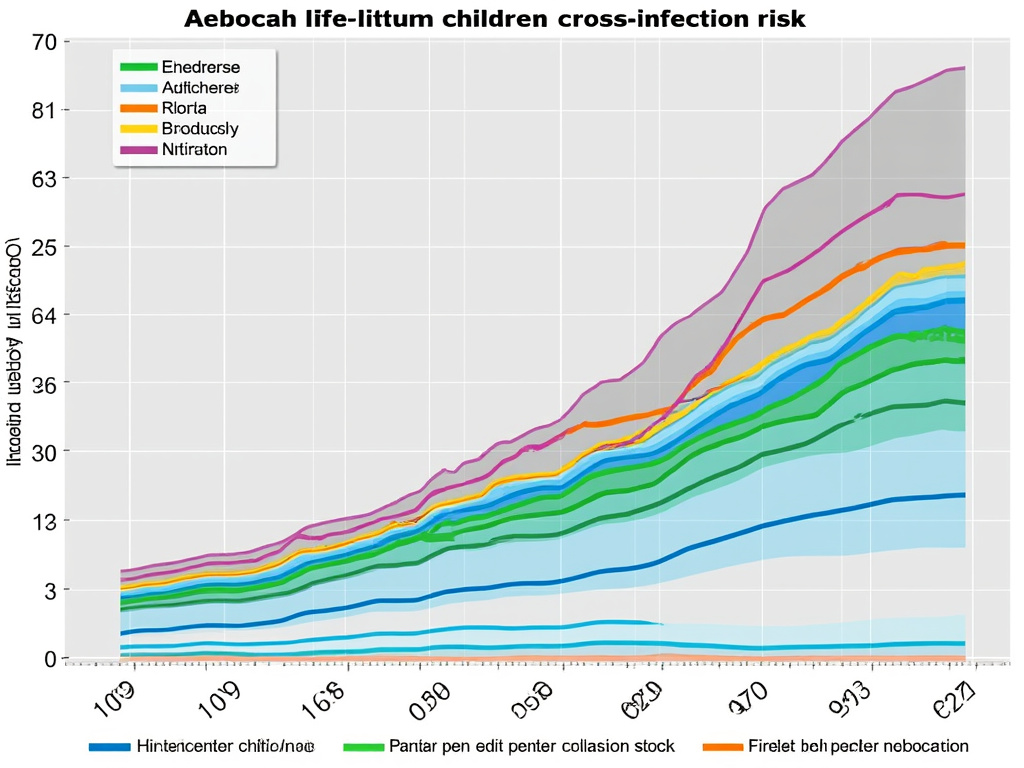
EAP BLOG – COVID-19 SERIES (#5) | ICELAND’S DATA ON THE INFECTIVITY OF CHILDREN CROSS-INFECTION RISK
COVID-19 SERIES (#5) Iceland’s data on the infectivity of children cross-infection risk. At the start of the COVID-19 pandemic, there was uncertainty with regards to how badly children would be affected by the novel coronavirus. We feared the worst…

EAP BLOG – COVID-19 SERIES (#4) | CRITICAL ETHICAL DECISIONS ON THE FRONTLINE – LEARNING FROM THE ADULTS
COVID-19 SERIES (#4) Critical Ethical Decisions on the Frontline – Learning from the Adults. Italy is facing a massive burden from the coronavirus disease 2019 (COVID-19) pandemic. Since Feb 21, 2020, when the first case of COVID-19 was recorded in…

YOUNG EAP BLOG – COVID-19 SERIES (#1) | SARS-COV-2 INFECTION IN CHILDREN – REVIEW OF THE CURRENT SITUATION
COVID-19 SERIES (#1) SARS-CoV-2 Infection in Children – Review of the Current Situation. “This is a time for facts, not fear. This is a time for rationality, not rumours. This is a time for solidarity, not stigma” – Dr. Tedros Adhanom Ghebreyesus,…

EAP BLOG – COVID-19 SERIES (#2) | THE CHANGING FACE OF MEDICINE
COVID-19 SERIES (#2) The Changing Face of Medicine. Pediatrics, the art of healing children, is undergoing a tremendous transformation in the COVID 19 days. The basic elements of the profession – listening to the mother, inspecting the child…

EAP BLOG – COVID-19 SERIES (#3) | SUFFER THE LITTLE CHILDREN?
COVID-19 SERIES (#3) Suffer the Little Children? This is the fourth in a series of Dr. Rob Ross Russell’s brief musings on aspects of the COVID-19 crisis. They are all my personal views and should not for a moment be interpreted as advice or dogma –…

YOUNG EAP BLOG | RARE DISEASES, EVERYDAY EFFORTS
February 2020 Rare Diseases, Everyday Efforts. February 29th is Rare Disease Day. EAP has decided to dedicate this month’s blog to the challenges of rare diseases in paediatrics across Europe. the situation In Europe, a rare disease is defined as one…

YOUNG EAP BLOG | TOWARDS THE HARMONISATION OF PAEDIATRIC TRAINING IN EUROPE
January 2020 Towards the harmonisation of paediatric training in Europe. January 24th is the International Day of Education, so it seemed fitting that EAP has decided to dedicate this month’s blog to the educational challenges of paediatric training…

YOUNG EAP BLOG | ACCESS TO MEDICINES FOR CHILDREN
November – December 2019 Access to medicines for children. What’s the situation and how does this affect children? Millions of children still die every year before the age of 5, worldwide, due to treatable conditions. Poor access to essential medicines…


