#PowerSafely
An EAP-Duracell Partnership
#PowerSafely Campaign
🔋 Button Battery Awareness Day – 12 June
On 12 June the European Academy of Paediatrics (EAP) joins the international paediatric community in marking Button Battery Awareness Day.
Every year, thousands of children are injured or hospitalised due to accidental button battery ingestion — a growing threat that demands urgent attention and action.
As part of our commitment to child safety, the EAP reaffirms its call for:
🔒 Mandatory child-proof compartments in all devices containing button batteries
📦 Child-resistant packaging for all coin batteries sold across Europe
📣 We also encourage health professionals, parents, and caregivers to explore the excellent awareness resources made available by our affiliated association, European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN).
Let’s continue working together to protect children and power safely.

#PowerSafely
An EAP-Duracell Partnership
The European Academy of Paediatrics (EAP) and the battery manufacturer Duracell have launched a joint campaign to drive awareness of the dangers of swallowing coin batteries, in particular related to Lithium Coin batteries, for children. To help keep families safe, they are joining forces to “power safely”. Together, they are educating parents and caregivers about the dangers of swallowing lithium coin batteries – and how to prevent it.
Brussels/Geneva, 12 November 2024 – The European Academy of Paediatrics (EAP) and Duracell are extending their strategic partnership to continue their joint #PowerSafely campaign for a further three-year period, from 2025 onwards.
The joint objective of EAP and Duracell remains a significant reduction of the risk of Lithium Coin cell ingestion, and the number of actual cases. To that end, the partners continue to drive the awareness about prevention among parents and health care professionals. They will also address paediatricians, particularly EAP members, and other practitioners to improve their knowledge about detection, treatment and cure.
EAP and Duracell will also reinforce their public advocacy across Europe for the collection of reliable statistical data on battery ingestion by children hospital admissions etc. They are calling upon Eurostat and all national statistical offices to introduce the collection and publication of structured data related to battery ingestion incidents, particularly among young children.
“We are extremely happy to continue collaborating with Duracell to help minimise the risks of battery ingestions,” says Berthold Koletzko, President of the European Academy of Paediatrics. “As part of our joint #PowerSafely campaign, we are launching a series of infographics called ‘Protect Your Children’ which we share in 15 languages. Our members are invited to hang up the information in their waiting rooms i.e., a prime location to reach parents and caregivers.” “We also developed a master presentation for health care professionals to advise on detection, first aid and further treatment of battery ingestion.”
Javier Hernandez Reta, President Duracell Europe & Africa, adds, “We are proud about partnering with the leading paediatric association in Europe. While EAP’s expertise about child health and paediatric care is outstanding, we are contributing to the partnership through our unique knowledge about battery innovation for the benefit of child safety and our understanding of consumer attitudes.”
“A key criterion for our success will be the collection of reliable data about the ingestion of Lithium Coin cells. Such multi-year statistics will form a solid basis to understand the full magnitude of the issue, as well as the effectiveness of innovative solutions.”
#PowerSafely Campaign
We invite you to use the materials below as a part of the EAP-Duracell #PowerSafely campaign.
#PowerSafely Social Media Campaign
We have created #PowerSafely banners for dissemination on social media. We encourage use of the hashtag #PowerSafely for better visibility.
Download the banner and logo in your language here
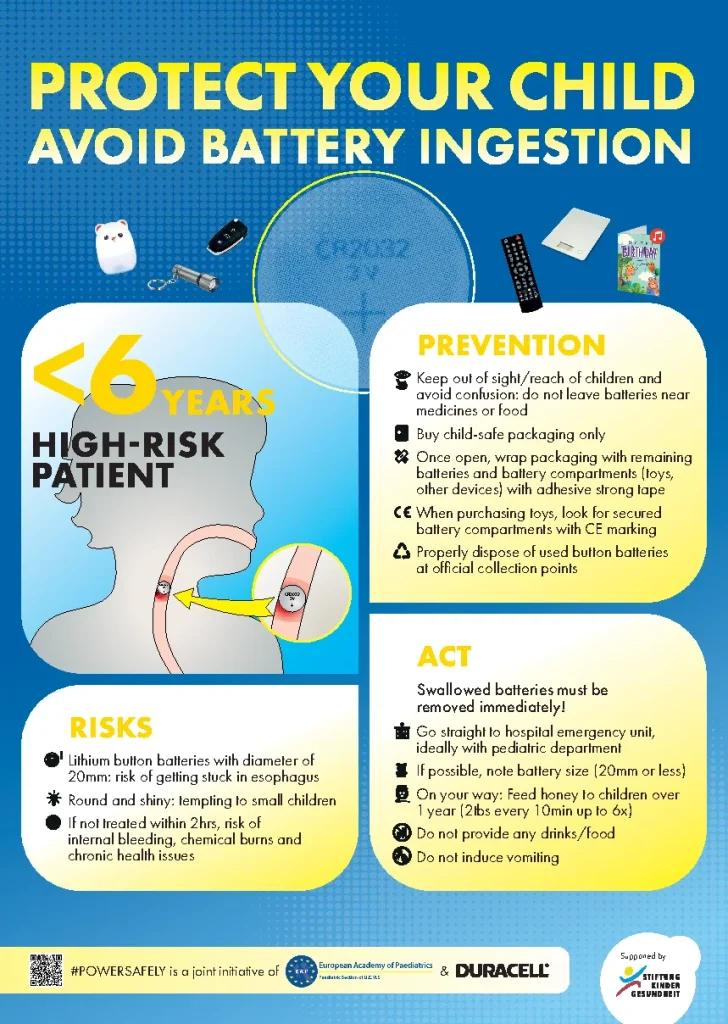
Waiting Room Infographic
We have made an infographic intended for display in waiting rooms and offices. Don’t hesitate to send round to surgeries and clinics in your country.
Download the infographic here
Press Release
Read the EAP Press Release to drive awareness of the dangers of coin battery swallowing, in particular related to Lithium Coin batteries, for children
Read the Presss release here
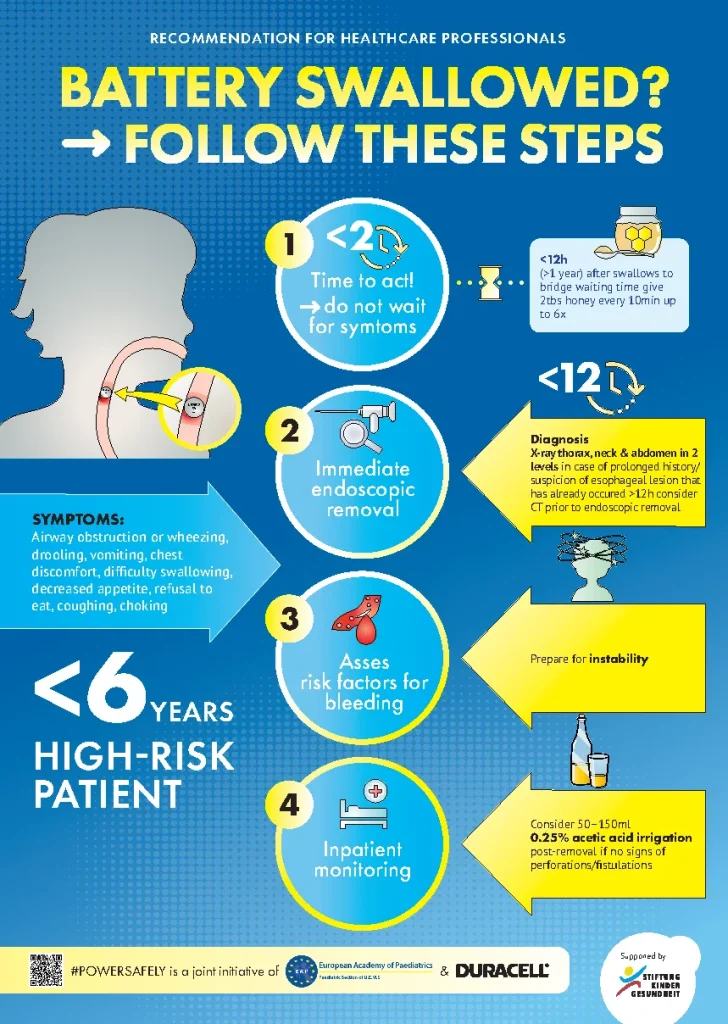
Presentations & infographics for healthcare professionals and partents offers guidance on the detection, first aid, and treatment of battery ingestion. These are available in multiple languages!
The #Powersafely campaign promotes
Awareness
We have created #PowerSafely banners for dissemination on social media. We encourage use of the hashtag #PowerSafely for better visibility.
Prevention
Preventive measures that can be taken to ensure your kids don’t swallow button batteries.
Response
What to do if your child swallows a button battery.